Abstract
Key message
High-frequency clonal seeds and near-normal fertility were obtained by engineering synthetic apomixis in hybrid rice.
Abstract
The one-line strategy, with the advantage of unnecessary seed production, is the final stage for the hybrid rice development and can be achieved through the fixation of heterosis via artificially inducing apomixis. Recently, synthetic apomixis has been generated in rice by combining MiMe (Mitosis instead of Meiosis) with either the ectopic expression of BABY BOOM (BBM1 or BBM4) or mutation of MATRILINEAL (MTL), resulting in over 95.00% of clonal seeds. However, the frequency of clonal seeds was only 29.20% when AtDD45 promoter was used to drive BBM1. In addition, achieving both a high frequency of clonal seeds and near-normal fertility simultaneously had been elusive in earlier strategies. In this study, using AtDD45 promoter to drive BBM1 expression in combination with the MiMe mutant resulted in the apomixis frequency as high as 98.70%. Even more, employing fusion promoters (AtMYB98_AtDD1_OsECA1-like1) to drive WUS expression in combination with pAtDD45:BBM1 and MiMe could produce clonal seeds at rates of up to 98.21%, the highest seed setting rate reached to 83.67%. Multiple-embryos were observed in clonal lines at a frequency ranging from 3.37% to 60.99%. Transmission of the high frequency of apomixis through skipped generations (atavism) was identified in two clonal lines, even though it remained stable in the majority of clonal lines. These findings significantly advance the pursuit of fixed heterosis in rice through synthetic apomixis, edging closer to its agricultural application.
Similar content being viewed by others
Introduction
Hybrid rice (Oryza sativa L.), with significant vigor in traits including yield, adaptability, and biotic and abiotic stress tolerance, have broken the yield ceiling of their inbred counterpart and have been extended exclusively in China and abroad (Birchler et al. 2010). Since Professor Longping Yuan’s team discovered O. rufipogon, a wild male sterile plant, in Hainan Province, China, in 1970, and developed successfully hybrid rice in 1973 (Yuan 1977), China has managed to feed nearly 20% of the world’s population with less than 9%of the world’s arable land with benefit from this technology. Outside of China, hybrid rice has also been introduced to nearly 70 countries across five continents, e.g., America, Vietnam, Pakistan, India, Philippines. However, hybrid seed production is costly and laborious (Grossniklaus et al. 2004), because F1 hybrid seeds must be renewed at every crop season. In 1987, Longping Yuan proposed three phases of the development of hybrid rice breeding, each using a particular methodology: the three-line method or Cytoplasmic Male Sterility (CMS) system; the two-line method or Thermosensitive or Photoperiod-sensitive Genic Male Sterile (TGMS or PGMS) system; the one-line method or apomixis system (Yuan 1987). The former two approaches have been most successfully applied in hybrid breeding (Mou 2016), but the one-line method is still under investigation.
Until recent years, an alternative to male sterility systems has emerged by engineering synthetic apomixis in plants which makes one-line hybrid rice possible. Sundaresan’s team ectopically expressed the BBM1 gene in rice egg cells in combination with MiMe and obtained approximately 29.20% of clonal seeds (Khanday et al. 2019). Guiderdoni’s team produced more than 95.00% of clonal seeds using MiMe mutants and AtECS promoter-driven BBM1 in a single step (Vernet et al. 2022). Kejian Wang’s team obtained 5.23% of clonal seeds by simultaneously editing REC8, PAIR1, OSD1, and MTL genes in hybrid rice (Wang et al. 2019), and three years later, they used the same strategy to discover the descendant clonal plants remained stable (Liu et al. 2023). In 2023, Kejian Wang’s team obtained a high seed-setting rate (range 80.90–86.10%) of clonal diploid plants by combining MiMe with ectopic expression of BBM4 in egg cells (Wei et al. 2023). Although the above studies represented important breakthroughs, the high clonal seeds-inducing rate and the high seed-setting rate have not yet been achieved simultaneously.
In this study, we introduced all-in-one T-DNA constructs into commercial hybrid rice, combined MiMe with ectopic expression of BBM1, which was driven by AtDD45 promoter or fusion promoter, a high frequency of apomixis, multiple-embryos, and near-normal fertility were obtained. Atavism (transmission through skipped generations) of the high frequency of apomixis was identified in two clonal lines, while the majority of clonal lines remained stable.
Materials and methods
Plant materials and growth conditions
Commercial cultivars Yongyou4949 (YY4) (Japonica/indica hybrid rice released in 2016 in China) were used in this study. Transgenic plants (T1, T2, T3, T4, and T5 generations), YY4 hybrid, and F2 progenies were grown as usual.
The seeds were randomly sampled and put into seed bags, placed in a covered foam box filled with sterile water, and soaked at 37 °C for 24 h. Then, the seeds were wrapped with a moist towel and cultivated for 2–3 days. The seeds were then placed in a petri dish and covered with three layers of sterilized filter paper. Afterward, an appropriate amount of sterile water was added, and germination was monitored after the seeds were cultivated in light at 28 °C for 6 days.
Plasmid construction and rice transformation
A CRISPR/Cas9 knockout expression vector p24MiMe (sgMiMe), designed to generate MiMe mutants by editing the OsOSD1 (Os02g37850), PAIR1 (Os03g01590), and OsREC8 (Os05g50410) genes (the target sites were listed in Table S5), was constructed using a method described previously (Ma et al. 2015). The expression cassette, including the coding sequence (CDS) of embryogenic gene BBM1 driven by the egg-specific promoter AtDD45 and terminated by Nos terminator (Steffen et al. 2007), was synthesized and cloned into the p24MiMe vector, the produced sgMiMe_pAtDD45:BBM1 was renamed p63C vector (GenScript Biotech Corp Co., Ltd., Nanjing). The p94C (‘sgMiMe’_pAtDD45:BBM1) vector with modified gRNAs of three MiMe genes was constructed to increase editing efficiency (Edgene Biot Co., Ltd., Wuhan, the target sites were listed in Table S5). The expression cassette, containing the fusion promoters (Arabidopsis MYB98 synergid cell-specific promoter; Arabidopsis DD1 antipodal cell-specific promoter; rice ECA1-like1 egg cell-specific promoter) (Susaki et al. 2021) driving the AtWUS CDS and terminated by PINII terminator, was synthesized and cloned into the p94C vector, the produced ‘sgMiMe’_pAtMYB98+ pAtDD1+ pOsECA1-like1:WUS_pAtDD45:BBM1 was renamed p95C vector (GenScript Biotech Corp Co., Ltd., Nanjing). These vectors were subsequently introduced into EHA105 competent cells and transformed into YY4 hybrid, afterwards, calli induction and plant regeneration were performed according to methods described previously (Xia et al. 2019).
Identification of mutants
Genomic DNA from the leaf of transgenic and control plants was extracted using the cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle 1990). PCR amplification of the OsOSD1, PAIR1, and OsREC8 genes was performed using 2 × Taq PCR Mix (primers were listed in Table S6). The amplified fragments were sequenced by the Sanger method, and the sequencing results were compared and analyzed by Sequencher 5.4.6 software (Tsingke Biotechnology Co., Ltd., Beijing).
Genotyping by pinpoint sequencing of multiplex PCR products
Batch Primer 3 software was used to design specific primers for target regions. These specific primers were used to amplify target interval sequences via multiple PCR, and the amplified target interval sequences were captured and enriched to construct a sequencing library. The samples were then sequenced via high-throughput sequencing. The raw data were filtered with FastQC 0.11.4 software to obtain clean reads, and the Burrows-Wheeler Alignment tool (BWA) was used to compare the clean sequences with the rice reference genome sequence. The single-nucleotide polymorphisms (SNPs) among the clean reads were detected and filtered by FreeBayes software (Higentec Co., Ltd., Changsha).
Flow cytometry determination
The ploidy of transgenic and control plants was determined (Ploidy Expert, Beijing) by estimating nuclear DNA content using flow cytometry (NovoCyte 3005), following the guidelines of the reagent kit (CyStainTMPI Absolute P).
Agronomic traits determinations and data analysis
Transgenic plants were grown at the Sanya transgenic base in Hainan and Changsha transgenic base in Hunan, China. The following agronomic traits were investigated at mature stage: plant height, number of panicles, panicle length, seed setting rate (filled grains/total grains), one-thousand grain weight, grain length, and grain width. For these agronomic traits, we used Duncan’s test with a significance of 0.05%. The SPASS software was used to analyze data.
Cytological observations of rice embryo sacs
Wild-type and transgenic florets at different stages were collected and fixed in FAA solution, kept at room temperature for 24 h, washed with 50% ethanol, and then stored at 4 °C in 70% ethanol. Embryo sacs were prepared according to a previous method (Huang et al. 2017) and were scanned by a confocal laser scanning microscope (Zeiss LSM880) with excitation wavelength 543 nm and the emission wavelength 550–630 nm.
Results
High efficiency of apomixis and near-normal fertility resulted from the ectopic expression of BBM1 driven by AtDD45 or fusion promoter combined with MiMe in hybrid rice
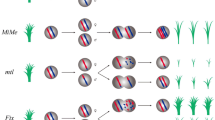
To generate clonal seeds through apomixis, four T-DNA constructs were transformed into the commercial hybrid rice YY4 using an Agrobacterium-mediated method. The first construct sgMiMe (p24MiMe) was designed to knock out OsOSD1, PAIR1, and OsREC8 genes via CRISPR/Cas9, leading to mitosis instead of meiosis (MiMe) and production of diploid clonal gametes (Khanday et al. 2019; Mieulet et al. 2016; Wang et al. 2019) (Fig. 1a). The second construct sgMiMe_pAtDD45:BBM1 (p63C) contained the BBM1 gene driven by the Arabidopsis egg-cell-specific promoter AtDD45 (pAtDD45:BBM1) created in p24MiMe background, inducing the autonomous embryonic development in MiMe mutant (Fig. 1b). The third construct ‘sgMiMe’_pAtDD45:BBM1 (p94C) was created by altering the gRNAs of three MiMe genes in the p63C background to increase editing efficiency (Fig. 1c). The fourth construct ‘sgMiMe’_pAtMYB98_pAtDD1_pOsECA1-like1:WUS_pAtDD45:BBM1 (p95C), created in p94C background, in addition carried WUS driven by the fusion promoter (AtMYB98_AtDD1_OsECA1-like1) (Susaki et al. 2021), a cassette triggering apomixis from synergid cells and antipodal cells beside of egg cell to enhance the efficiency of apomixis (Fig. 1d). In the T0 transgenic populations, 3 (n = 63, 4.76%), 10 (n = 235, 4.26%), 29 (n = 42, 69.05%), and 25 (n = 42, 59.52%) transformants were identified as triple mutants in the p24MiMe, p63C, p94C, and p95C, respectively. This suggests that modifying the gRNA of the three MiMe genes can significantly improve editing efficiency in hybrid rice.
Ploidy, genotype, and phenotype of progeny plants of transformations in hybrid rice. a Schematic diagram of the T-DNA structure sgMiMe (p24MiMe) targeting OsOSD1, PAIR1, and OsREC8 (MiMe). pZmUBIQUITIN1, Zea mays UBIQUITIN 1 promoter; Cas9, Cas9 gene; Nos, nopaline synthase terminator. b Schematic diagram of the T-DNA construct sgMiMe_pAtDD45:BBM1 (p63C) containing the MiMe and pAtDD45:BBM1 gene expression cassettes. pAtDD45, egg cell-specific from Arabidopsis; BBM1, BBM1 gene. c Schematic diagram of the T-DNA construct ‘sgMiMe’_pAtDD45:BBM1 (p94C). Compared to p63C, p94C was created by altering the gRNAs of three MiMe genes to increase editing efficiency. d Schematic diagram of the T-DNA construct ‘sgMiMe’_pAtMYB98_pAtDD1_pOsECA1-like1:WUS_pAtDD45:BBM1 (p95C). pAtMYB98, synergid cell-specific from Arabidopsis; pAtDD1, antipodal cell-specific from Arabidopsis; pOsECA1-like1, egg cell-specific from rice; WUS, WUS gene from Arabidopsis. PinII, the terminator of proteinase inhibitor II. e Plant morphology of line G23-6 and line G23-18 (p63C) were compared to wild-type. The sexual tetraploid plant displayed high sterility. Scale bars, 20 cm. f Comparison of the T1 seeds between line G23-6 (p63C) and wild-type. The seed of tetraploid showed a large size with awns. Scale bars, 2 mm. g Representative ploidy analysis of a tetraploid and diploid according to flow cytometry. h The frequency of clonal seeds and seed-setting of p95C events. In the T1 clonal lines of p95C, a high seed-setting rate was observed, but the clonal seed rate was low. Conversely, there was a high frequency of clonal seeds with a low seed-setting rate. Details were provided in Table 2. i Plants morphology of line HW14 (p94C) and line CL3 (p95C) were compared to wild-type. Scale bars, 20 cm. j Comparison of the panicles between T1 diploid clonal plants (p94C and p95C) and wild-type. Scale bars, 5 cm. k Genotyping by pinpoint sequencing of wild-type, F2 sexual progenies, T1 clonal lines of p94C and p63C. Compared to wild-type, the site similarity of F2 progeny plants ranged from 43.62% to 56.44% resulting from meiotic crossovers, while diploid clonal plants maintained a site similarity of more than 95.00% due to the absence of meiotic division
For examining ploidy and seed-setting rate, a crucial agricultural trait for apomixis, 3, 2, 5, and 11 MiMe mutants were selected from the fertile T0 transformants of the p24MiMe, p63C, p94C, and p95C, respectively. Clonal diploid plants are easily distinguishable by phenotypic observation from tetraploid plants, characterized by high sterility and large, awned grains (Fig. 1e–f), which could be confirmed by flow cytometry analysis (Fig. 1g). First, a conflicted relationship was observed between the frequency of clonal seeds and seed-setting, with clonal lines with high frequency of seed-setting rate exhibiting low frequency of clonal seeds, and vice versa (Fig. 1h, Table 2B). This phenomenon is prevalent in all clonal lines we have obtained (Table 2). Fortunately, we can obtain the relatively high frequency of clonal seeds in the case of ensuring the seed-setting rates.
All progenies of the p24MiMe T0 events were determined to be tetraploid (Table 1), a result of diploid gamete fusion. If parthenogenesis is triggered through the expression of BBM1, driven by AtDD45 in combination with MiMe, clonal seeds of the T1 progeny should be shown as previously at a frequency of 10–30% (Khanday et al. 2019). Exceeding our expectations, sgMiMe_pAtDD45:BBM1 (p63C) transformants produced over 90.00% diploid clonal plants, exhibiting seed-setting rates were 44.88% and 51.35% (Table 1, Table 2A). As for the transformants of p94C, the maximum frequency of clonal seeds reached 97.50% (line HW7), while the peak seed-setting rate was 68.16% (line HW14). Notably, the line HW14 showed the most optimal comprehensive traits, with a clonal seeds rate of 90.79% and a seed-setting rate of 68.16% (Table 2A). In the T0 transformants of p95C, 11 of the 25 were analyzed, the maximum frequency of clonal seeds reached 98.21% (line CL10), and the peak seed-setting rate was 83.67% (line CL3), which was comparable to that of wild-type (88.65%, range 84.17–93.10%). Notably, the line CL3, exhibiting superior comprehensive traits, demonstrated 46.48% of clonal seeds and 83.67% of seed-setting rate (Table 1, Table 2B). The results suggest that an unexpectedly high frequency of clonal seeds can also be yielded using AtDD45 promoter to drive BBM1 expression or fusion promoter in combination with the MiMe.
To determine if heterozygosity was maintained in the progenies of apomicts, pinpoint sequencing genotyping was conducted in the wild-type, F2 progenies, and five T1 clonal lines (Fig. 1k). Compared to wild-type, the site similarity in F2 progenies ranged from 43.62% to 56.44%, arising from meiotic crossovers. In contrast, clonal lines exhibited a site similarity of up to 98.92%, due to the absence of meiotic division. The sequencing result indicates that the T1 clonal lines have retained their heterozygous genotypes, confirming their clonal nature.
The phenotypes are important to investigate whether clonal lines are suitable for commercial applications. We selected four clonal lines of the p94C, all of which yielded more than 80.00% diploid clonal plants (T1), to explore if these clonal plants maintain the F1 hybrid (wild-type) phenotype. The gross phenotype of T1 clonal plants displayed similar traits such as plant height, number of panicles, and panicle length with the F1 hybrid plants (Fig. 1i–j). Phenotypes were more thoroughly examined in T1 progenies in four p94C transformants, grown with control wild-type. No significant differences were detected in the number of panicles and grain width when comparing the control F1 hybrid with the clonal plants. The few exceptions comprised a significantly higher plant height and longer grain length in line HW14 and lighter one-thousand grain weight in line HW7 and shorter panicle length in all clonal lines except line HW14 (Table S2). These differences may arise from somatic mutations and environmental situations. In summary, the assessment of observable characteristics in progenies from p94C transformants reveals a consistent phenotype closely resembling that of the F1 hybrid.
Altogether, using AtDD45 and fusion promoter can yield one-line hybrid rice with high-frequency clonal seeds and near-normal fertility in combination with the MiMe. The genotype and phenotype are retained in the offspring of clonal plants.
High frequency of multiple-embryos resulted from the ectopic expression of BBM1 combined with MiMe
In the clonal lines, there was a phenomenon where a single seed with multiple plumule axis, typically two or three, which we have termed multiple-embryos (Fig. 2a–d). Among the T0 transformants, two of the p63C, four of the p94C, and six of the p95C exhibited over 20.00% of multiple-embryos, and this phenomenon was maintained in descendants (Table 3, Table S3). Significantly, line HW7 (p94C) displayed a high frequency (60.99%) of multiple-embryos (Table 3). In addition, all these transformants also showed a high penetrance of apomixis (Table 1). Unsurprisingly, no multiple-embryos were observed in MiMe-only mutants (line MM-9, MM-10 and MM-11 from p24MiMe). To determine if the ectopic expression of the BBM1 gene in combination with MiMe induced multiple-embryos, we examined four transformants without the MiMe mutation (lines G23-5, G23-11, G23-13, and G23-16 from p63C). Fewer than 4.00% of multiple-embryos were exhibited in the transformants (Fig. 2e, Table 3), which was consistent with our expectations. These findings show that ectopic expression of BBM1 can induce fewer multiple-embryos, but the high penetrance of multiple-embryos resulted from the combination of ectopic expression of BBM1 with MiMe.
Ploidy, phenotype, and embryo sac observation of multiple-embryos. a, b Discovery of multiple-embryos. In terms of morphology, the multiple-embryos displayed a single seed with two or three plumule axes. Scale bars, 2 mm. c, d Morphology of multiple-embryos. At 15 d after germination, the morphology of multiple-embryos showed two or three seedlings. Scale bars, 2 cm. e Discovery of multiple-embryos in p63C transgenic plants (without MiMe mutation). Germination of 6 d-old twin-embryos. Scale bars, 2 mm. f Flow cytometry DNA histograms for ploidy determination of multiple-embryos (2n/n). One was a sexual diploid showing a 2n peak, and the other was an asexual haploid showing a 1n peak. g Characterization of haploids. Differences in height between wild-type and haploid seedlings derived from p63C transformants (without MiMe mutation) at 6 d after germination. Scale bars, 2 mm. h Comparative analysis of the morphology of diploid and haploid floral organs. Scale bars, 1 mm. i Comparison of the morphology across different ploidy (2n/n) in multiple-embryos. Two seedlings were planted separately because the asexual haploid had limited growth potential. Scale bars, 20 cm. j Comparison of the main spikes in multiple-embryos (2n/n). Compared to the sexual diploid, the panicle of the asexual haploid showed infertile seeds. Scale bars, 5 cm. k Ploidy analysis of the multiple-embryos (2n/4n) according to flow cytometry. One of the multiple-embryos was diploid (asexual), showing a 2n peak; and the other was tetraploid (sexual), showing a 4n peak. l Comparison of the morphology across different ploidy (2n/2n, 4n/2n, and 4n/4n) in twin-embryos. Scale bars, 20 cm. m Comparison of the morphology across different ploidy (4n/2n/2n and 2n/2n/2n) in triple-embryos. Scale bars, 20 cm. n Representative embryos at 48 h and 72 h after flowering (HAF) from wild-type (I, II) and multiple-embryos (III, IV). Compared to wild-type, multiple-seedlings showed two embryos formed at the micropyle. Ge globular embryos, Pe pyriform embryos. Scale bars, 2 mm
The ploidy level of multiple-embryos was determined through phenotypic observation and confirmed using flow cytometry. Ploidy analysis showed that twin-embryos from transformants without MiMe mutation were 2n/n chimeric seedlings (Fig. 2f). Compared to sexual diploid plants, asexual haploids showed typical haploid characteristics, including sterile and small floral organs (Fig. 2g–j). In contrast, twin-embryos from transformants (BBM1 expression with MiMe mutation) were either both 2n, both 4n, or 4n/2n chimeric seedlings (Fig. 2k–l). The triple-embryos exhibited 4n/2n/2n or 2n/2n/2n (Fig. 2m). These results suggest that 2n seedlings may arise from apogamy while 4n seedlings result from zygotes, indicating a potential to increase the frequency of clonal seeds via apogamy embryogenesis.
We employed whole-mount stain-clearing laser scanning confocal microscopy (WCLSM) to observe the development of embryo sacs in diploid clonal plants. Notably, at 48 and 72 h after flowering (HAF), we observed two embryos forming at the micropyle in embryo sacs. In contrast, the control wild-type displayed only a single embryo (Fig. 2n). This suggests that the presence of multiple seedlings might arise from two or more embryos in the same embryo sac, sharing a common endosperm.
High efficiency of apomixis was atavism (transmission through skipped generations)
The stability of the synthetic apomixis system over multiple generations was discovered in the majority of transgenic lines. In the T2 plants of line G23-6 (p63C), the determination of ploidy level revealed more than 90.00% of clonal plants, consistent with observations in T1 plants (Table 1, Table S3). Similarly, among the T2 clonal lines of p94C, 4 out of the 5 apomicts exhibited clonal seeds frequencies ranged from 77.57% to 98.70%, which were also consistent with the performance of their T1 plants (Table 1, Table S3). The seed-setting rates of T2 diploid clonal plants from p63C (range 55.05–56.92%) and p94C (range 30.89–66.10%) transformants were corresponding to observations in T1 plants (Table 2A). Altogether, both apomixis and seed-setting rates exhibited stability across generations in the majority of transgenic lines.
Interestingly, the phenomenon of atavism (transmission across skipped generations) was observed in two lines based on the frequency of apomixis. In line G23-18 (p63C), the clonal seeds frequencies for T1 through T5 plants were 96.55%, 15.18%, 96.75%, 13.54%, and 17.56%, respectively (Table 1, Table S3). Notably, the frequency was increased in T1 and T3 plants but decreased in T2 and T4 plants. This atavism phenomenon was absent in the T5 generation (Fig. 3a, b). The seed-setting rate of T1 to T5 clonal plants from line G23-18 (p63C) was significantly lower than that of the wild-type (76.26%), with the average seed-setting rates of 51.35%, 56.92%, 49.64%, 58.82%, and 55.88%, respectively (Fig. 3c). The similar result was also observed in line HW10 (p94C), with the apomixis frequencies of 66.25% and 38.75% (Table 1, Table S3), and the seed-setting rates of 66.25% and 56.21% in T1 and T2 plants (Table 2), respectively. These findings show a trend of high frequency of clonal seeds in alternate generations, referred to as atavism.
The phenomenon of atavism. a Frequency of clonal seeds in T1, T2, T3 and T4 plants of line G23-18 (p63C). b Comparison of frequency of clonal seeds from T1 to T5 plants in line G23-18 (p63C). In the T1, T2, T3, T4, and T5 generations, the average frequency of diploid clonal plants was 96.55%, 15.18%, 96.75%, 13.54%, and 17.56%, respectively. Scale bars, 20 cm. Details were provided in Table S3. c Seed-setting rate in T1 to T5 clonal plants of line G23-18 (p63C). In the T1, T2, T3, T4 and T5 generations, the average seed-setting rate was 51.35%, 56.92%, 49.64%, 58.82%, and 55.88%, respectively
Discussion
One-line hybrid rice is Professor Longping Yuan’s living will and unfinished business. In 1987, he proposed a development strategy for hybrid rice: from a three-line to a two-line, and later on to a one-line, paralleling a shift from complexity to simplicity (Yuan 1987). The one-line hybrid rice could be achieved by synthetic apomixis, which can lead to the fixation of heterosis and reduce the cost of hybrid rice seed production. A previous study using the promoter AtDD45 to drive BBM1 just reached 29.20% synthetic apomixis in the inbred cultivar Kitaake (Khanday et al. 2019). In this study, we used AtDD45 promoter to drive BBM1 expression in combination with the MiMe, resulting in the production of high-efficiency clonal seeds at a rate of 98.70% in hybrid rice YY4 (japonica/indica). In addition, we designed the construct ‘sgMiMe’_pAtMYB98_pAtDD1_pOsECA1-like1:WUS_pAtDD45:BBM1, yielding clonal seeds frequencies of up to 98.21%. We also analyzed the differences in fertility between the control and clonal lines under a field trial. The clonal lines of p94C (range 33.73–68.16%) and p95C (range 15.75–83.67%) exhibited highly variable seed-setting rates under a field trial. The highest seed-setting rate was comparable to the control YY4 hybrid (88.65%). Specifically, the lines with the most optimal comprehensive traits were line HW14 (p94C) and line CL3 (p95C), the seed-setting rate of line HW14 and line CL3 was 68.16% and 83.67%, respectively. Meanwhile, the production of clonal seeds reached 90.79% for line HW14 and 46.48% for line CL3. These results indicate the potential of synthetic apomixis in agricultural applications.
In addition, we observed a conflicting relationship between the frequency of seed-setting and clonal seeds in diploid clonal plants, where clonal lines with a high frequency of seed-setting rate exhibited a low frequency of clonal seeds, and vice versa. We speculate that during the initiation of embryo autonomous development through parthenogenesis of synthetic apomixis, signals for cell division and differentiation are released at high penetrance. Consequently, other cells in the embryo sac, such as the synergid cells, perceive these signals as an indication of completed fertilization, which will affect sperm cell entry into the embryo sac, subsequently influencing central cell fertilization and inhibiting endosperm formation, thereby leading to a reduced seed-setting rate. An autonomous endosperm may result in both normal seed formation and an increase in the seed-setting rate. Another strategy to increase the seed-setting rate is employing egg-cell-specific promoters, which activate expression at the exact time to induce embryogenesis without affecting central cell fertilization. We also propose an alternative way, sorting the clonal seeds, to address the conflict between the frequency of seed-setting and clonal seeds. In our research, we obtained some lines (e.g., line CL3) with near-normal seed-setting rates, and their apomixis efficiency was relatively low. Subsequently, we can separate the clonal seeds using pollen-specific gene switch system. This technology distinguishes non-fluorescent clonal seeds from red-fluorescent sexual seeds through photoelectric sorting, such that the clonal seeds could be selectively used for field planting (Cao et al. 2019). We have reported that clonal lines exhibited a high penetrance of multiple-embryos for the first time. Specifically, two embryos were observed in the embryo sac and this trait was stably inherited across multiple generations. No multiple-embryos were observed in MiMe-only mutants, and they were low frequency in the transformants without MiMe mutation from p63C. This indicate that the BBM1 gene is essential for the production of multiple embryos. MiMe mutants produced diploid gametes, and the ploidy detection of multiple-embryos was 2n/2n, 4n/4n, or 4n/2n chimeric plants, respectively. Ploidy detection of multiple-embryos in the transformants without MiMe mutation was 2n/n chimeric plants, due to haploid gametes. In the embryo sac, asexual haploid embryos exhibit reduced viability in competing with sexual diploid embryos. This could account for the observation that only the combination of MiMe and the ectopic expression of BBM1 results in the production of multiple-embryos at a high efficiency. We also detected haploids in the transformants without MiMe mutation with frequencies less than 8.33% (Table S4). We speculate that the formation of multiple embryos in clonal lines can be attributed to the following reasons: the first is embryo division, BBM1 protein activates the gene involved in embryo division; the second is that promoter AtDD45 is not only expressed in egg cells but also other cells, such as synergids, which may subsequently develop into embryos; the third is BBM1 protein activates the gene involved in cell division during egg cell formation resulted in two egg cells in the micropyle. As a result, one seed with two or three embryos, and germinating multiple-embryos, it is of great significance to the innovation and utilization of crop germplasm, with fewer seeds to obtain the same yielding.
The stability of clonal lines over generations has been previously demonstrated in previous reports (Vernet et al. 2022; Liu et al. 2023). Our study was consistent with previous research in the majority of lines. An intriguing phenomenon of atavism was observed in two lines, with high penetrance of apomixis in both the T1 and T3 generations, contrasting with low occurrences in the T2 and T4 generations. Notably, the atavism phenomenon disappeared in the T5 generation. This observation has not been previously reported and contradicts traditional genetic principles. Epigenetic control of gene expression may be responsible for this phenomenon, and further investigation into the underlying mechanisms is warranted.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Birchler JA, Yao H, Chudalayandi S et al (2010) Heterosis. Plant Cell 22:2105–2112. https://doi.org/10.1105/tpc.110.076133
Cao ML, Xia YM, Zhan YJ et al (2019) A seed sorting method for fixed plant heterosis. CN201910307717.3
Doyle JJT, Doyle JL (1990) Isolation of DNA from fresh tissue. Focus 12:13–15
Grossniklaus U, Spillane C, Curtis MD (2004) Apomixis technology development-virgin births in farmers’ fields? Nat Biotechnol 22:687–691. https://doi.org/10.1038/nbt976
Huang X, Peng X, Sun MX (2017) OsGCD1 is essential for rice fertility and required for embryo dorsal-ventral pattern formation and endosperm development. New Phytol 215:1039–1058. https://doi.org/10.1111/nph.14625
Khanday I, Skinner D, Yang B et al (2019) A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 565:91–95. https://doi.org/10.1038/s41586-018-0785-8
Liu CL, He ZX, Zhang Y et al (2023) Synthetic apomixis enables stable transgenerational transmission of heterotic phenotypes in hybrid rice. Plant Commun 4:100470. https://doi.org/10.1016/j.xplc.2022.100470
Ma XL, Zhang QY, Zhu QL et al (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8:1274–1284. https://doi.org/10.1016/j.molp.2015.04.007
Mieulet D, Jolivet S, Rivard M et al (2016) Turning rice meiosis into mitosis. Cell Res 26:1242–1254. https://doi.org/10.1038/cr.2016.117
Mou TM (2016) The research progress and prospects of two-line hybrid rice in China. CSB 61:3761–3769. https://doi.org/10.1360/N972016-01045
Steffen JG, Kang IH, Macfarlane J et al (2007) Identification of genes expressed in the Arabidopsis female gametophyte. Plant J 51:281–292. https://doi.org/10.1111/j.1365-313X.2007.03137.x
Susaki D, Suzuki T, Maruyama D et al (2021) Dynamics of the cell fate specifications during female gametophyte development in Arabidopsis. PLoS Biol 19:e3001123. https://doi.org/10.1371/journal.pbio.3001123
Vernet A, Meynard D, Lian QC et al (2022) High-frequency synthetic apomixis in hybrid rice. Nat Commun 13:7963. https://doi.org/10.1038/s41467-022-35679-3
Wang C, Liu Q, Shen Y et al (2019) Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes. Nature Biotechnol 37:283–286. https://doi.org/10.1038/s41587-018-0003-0
Wei X, Liu CL, Chen X et al (2023) Synthetic apomixis with normal hybrid rice seed production. Mol Plant 16:489–492. https://doi.org/10.1016/j.molp.2023.01.005
Xia YM, Tang N, Hu YY et al (2019) A method for mechanized hybrid rice seed production using female sterile rice. Rice 12:1–10. https://doi.org/10.1186/s12284-019-0296-8
Yuan LP (1977) Practice and theory of hybrid rice cultivation. SAS 1:27–31
Yuan LP (1987) Conception of breeding strategy for hybrid rice. Hybrid Rice 1:1–3. https://doi.org/10.16267/j.cnki.1005-3956.1987.01.001
Funding
This study was funded by the National Natural Science Foundation of China Regional Joint Fund (U23A20189), the Natural Science Foundation of Hunan Province (2023JJ40471), and the Agricultural Technology Innovation Foundation of Hunan Province (2023CX13 and 2023CX79).
Author information
Authors and Affiliations
Contributions
MC conceived this project and supervised the whole project. HD wrote the manuscript. JD and YX performed the experiments and wrote the manuscript. YW contributed to the hybrid rice transformation and data analysis. YZ analyzed data. JT detected the ploidy and analyzed data. NT contributed to the plant photography. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by Xian Sheng Zhang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dan, J., Xia, Y., Wang, Y. et al. One-line hybrid rice with high-efficiency synthetic apomixis and near-normal fertility. Plant Cell Rep 43, 79 (2024). https://doi.org/10.1007/s00299-024-03154-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00299-024-03154-6