Abstract
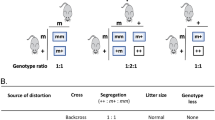
Cytosine base editing achieves C•G-to-T•A substitutions and can convert four codons (CAA/CAG/CGA/TGG) into STOP-codons (induction of STOP-codons, iSTOP) to knock out genes with reduced mosaicism. iSTOP enables direct phenotyping in founders’ somatic cells, but it remains unknown whether this works in founders’ germ cells so as to rapidly reveal novel genes for fertility. Here, we initially establish that iSTOP in mouse zygotes enables functional characterization of known genes in founders’ germ cells: Cfap43-iSTOP male founders manifest expected sperm features resembling human “multiple morphological abnormalities of the flagella” syndrome (i.e., MMAF-like features), while oocytes of Zp3-iSTOP female founders have no zona pellucida. We further illustrate iSTOP’s utility for dissecting the functions of unknown genes with Ccdc183, observing MMAF-like features and male infertility in Ccdc183-iSTOP founders, phenotypes concordant with those of Ccdc183-KO offspring. We ultimately establish that CCDC183 is essential for sperm morphogenesis through regulating the assembly of outer dynein arms and participating in the intra-flagellar transport. Our study demonstrates iSTOP as an efficient tool for direct reproductive disease modeling and phenotyping in germ cells of the founder generation, and rapidly reveals the essentiality of Ccdc183 in fertility, thus providing a time-saving approach for validating genetic defects (like nonsense mutations) for human infertility.
Similar content being viewed by others
References
Abbasi, F., Miyata, H., and Ikawa, M. (2018a). Revolutionizing male fertility factor research in mice by using the genome editing tool CRISPR/Cas9. Reprod Med Biol 17, 3–10.
Abbasi, F., Miyata, H., Shimada, K., Morohoshi, A., Nozawa, K., Matsumura, T., Xu, Z., Pratiwi, P., and Ikawa, M. (2018b). RSPH6A is required for sperm flagellum formation and male fertility in mice. J Cell Sci 131, jcs.221648.
Agarwal, A., Baskaran, S., Parekh, N., Cho, C.L., Henkel, R., Vij, S., Arafa, M., Panner Selvam, M.K., and Shah, R. (2021). Male infertility. Lancet 397, 319–333.
Aprea, I., Raidt, J., Hoben, I.M., Loges, N.T., Nothe-Menchen, T., Pennekamp, P., Olbrich, H., Kaiser, T., Biebach, L., Tuttelmann, F., et al. (2021). Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLoS Genet 17, e1009306.
Auguste, Y., Delague, V., Desvignes, J.P., Longepied, G., Gnisci, A., Besnier, P., Levy, N., Beroud, C., Megarbane, A., Metzler-Guillemain, C., et al. (2018). Loss of calmodulin- and radial-spoke-associated complex protein CFAP251 leads to immotile spermatozoa lacking mitochondria and infertility in men. Am J Hum Genet 103, 413–420.
Ben Khelifa, M., Coutton, C., Zouari, R., Karaouzène, T., Rendu, J., Bidart, M., Yassine, S., Pierre, V., Delaroche, J., Hennebicq, S., et al. (2014). Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet 94, 95–104.
Billon, P., Bryant, E.E., Joseph, S.A., Nambiar, T.S., Hayward, S.B., Rothstein, R., and Ciccia, A. (2017). CRISPR-mediated base editing enables efficient disruption of eukaryotic genes through induction of STOP codons. Mol Cell 67, 1068–1079.e4.
Chen, T., Bian, Y., Liu, X., Zhao, S., Wu, K., Yan, L., Li, M., Yang, Z., Liu, H., Zhao, H., et al. (2017). A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet 101, 459–465.
Conner, S.J., Lefièvre, L., Hughes, D.C., and Barratt, C.L.R. (2005). Cracking the egg: increased complexity in the zona pellucida. Hum Reprod 20, 1148–1152.
Coutton, C., Martinez, G., Kherraf, Z.E., Amiri-Yekta, A., Boguenet, M., Saut, A., He, X., Zhang, F., Cristou-Kent, M., Escoffier, J., et al. (2019). Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am J Hum Genet 104, 331–340.
Coutton, C., Vargas, A.S., Amiri-Yekta, A., Kherraf, Z.E., Ben Mustapha, S.F., Le Tanno, P., Wambergue-Legrand, C., Karaouzène, T., Martinez, G., Crouzy, S., et al. (2018). Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat Commun 9, 686.
Cuella-Martin, R., Hayward, S.B., Fan, X., Chen, X., Huang, J.W., Taglialatela, A., Leuzzi, G., Zhao, J., Rabadan, R., Lu, C., et al. (2021). Functional interrogation of DNA damage response variants with base editing screens. Cell 184, 1081–1097.e19.
Dang, L., Li, G., Wang, X., Huang, S., Zhang, Y., Miao, Y., Zeng, L., Cui, S., and Huang, X. (2020). Comparison of gene disruption induced by cytosine base editing-mediated iSTOP with CRISPR/Cas9-mediated frameshift. Cell Prolif 53, e12820.
Ding, X., and Schimenti, J.C. (2021). Strategies to identify genetic variants causing infertility. Trends Mol Med 27, 792–806.
Dong, F.N., Amiri-Yekta, A., Martinez, G., Saut, A., Tek, J., Stouvenel, L., Lorès, P., Karaouzène, T., Thierry-Mieg, N., Satre, V., et al. (2018). Absence of CFAP69 causes male infertility due to multiple morphological abnormalities of the flagella in human and mouse. Am J Hum Genet 102, 636–648.
Ernst, C., Eling, N., Martinez-Jimenez, C.P., Marioni, J.C., and Odom, D.T. (2019). Staged developmental mapping and X chromosome transcriptional dynamics during mouse spermatogenesis. Nat Commun 10, 1251.
Frock, R.L., Hu, J., Meyers, R.M., Ho, Y.J., Kii, E., and Alt, F.W. (2015). Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat Biotechnol 33, 179–186.
Gadadhar, S., Alvarez Viar, G., Hansen, J.N., Gong, A., Kostarev, A., Ialy-Radio, C., Leboucher, S., Whitfield, M., Ziyyat, A., Touré, A., et al. (2021). Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science 371, eabd4914.
Gahlay, G., Gauthier, L., Baibakov, B., Epifano, O., and Dean, J. (2010). Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science 329, 216–219.
Ghezraoui, H., Piganeau, M., Renouf, B., Renaud, J.B., Sallmyr, A., Ruis, B., Oh, S., Tomkinson, A.E., Hendrickson, E.A., Giovannangeli, C., et al. (2014). Chromosomal translocations in human cells are generated by canonical nonhomologous end-joining. Mol Cell 55, 829–842.
Gou, L.T., Gao, Y., Kang, J.Y., Wang, X., Chen, H., Hua, M.M., Li, Z., Li, D., Fu, X.D., Shi, H.J., et al. (2021). Reply to lack of evidence for a role of PIWIL1 variants in human male infertility. Cell 184, 1943–1944.
Gou, L.T., Kang, J.Y., Dai, P., Wang, X., Li, F., Zhao, S., Zhang, M., Hua, M.M., Lu, Y., Zhu, Y., et al. (2017). Ubiquitination-deficient mutations in human Piwi cause male infertility by impairing histone-to-protamine exchange during spermiogenesis. Cell 169, 1090–1104.e13.
Hartill, V.L., van de Hoek, G., Patel, M.P., Little, R., Watson, C.M., Berry, I.R., Shoemark, A., Abdelmottaleb, D., Parkes, E., Bacchelli, C., et al. (2018). DNAAF1 links heart laterality with the AAA+ ATPase RUVBL1 and ciliary intraflagellar transport. Hum Mol Genet 27, 529–545.
He, X., Liu, C., Yang, X., Lv, M., Ni, X., Li, Q., Cheng, H., Liu, W., Tian, S., Wu, H., et al. (2020). Bi-allelic loss-of-function variants in CFAP58 cause flagellar axoneme and mitochondrial sheath defects and asthenoteratozoospermia in humans and mice. Am J Hum Genet 107, 514–526.
Houston, B.J., Conrad, D.F., and O’Bryan, M.K. (2021a). A framework for high-resolution phenotyping of candidate male infertility mutants: from human to mouse. Hum Genet 140, 155–182.
Houston, B.J., Riera-Escamilla, A., Wyrwoll, M.J., Salas-Huetos, A., Xavier, M.J., Nagirnaja, L., Friedrich, C., Conrad, D.F., Aston, K.I., Krausz, C., et al. (2021b). A systematic review of the validated monogenic causes of human male infertility: 2020 update and a discussion of emerging gene-disease relationships. Hum Reprod Update 28, 15–29.
Jamsai, D., Lo, J.C.Y., McLachlan, R.I., and O’Bryan, M.K. (2014). Genetic variants in the RABL2A gene in fertile and oligoasthenospermic infertile men. Fertil Steril 102, 223–229.
Jia, K., Lu, Z., Zhou, F., Xiong, Z., Zhang, R., Liu, Z., Ma, Y., He, L., Li, C., Zhu, Z., et al. (2019). Multiple sgRNAs facilitate base editing-mediated i-stop to induce complete and precise gene disruption. Protein Cell 10, 832–839.
Jiao, S.Y., Yang, Y.H., and Chen, S.R. (2021). Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update 27, 154–189.
Kherraf, Z.E., Amiri-Yekta, A., Dacheux, D., Karaouzène, T., Coutton, C., Christou-Kent, M., Martinez, G., Landrein, N., Le Tanno, P., Fourati Ben Mustapha, S., et al. (2018). A homozygous ancestral SVA-insertion-mediated deletion in WDR66 Induces multiple morphological abnormalities of the sperm flagellum and male infertility. Am J Hum Genet 103, 400–412.
Kherraf, Z., Cazin, C., Coutton, C., Amiri-Yekta, A., Martinez, G., Boguenet, M., Fourati Ben Mustapha, S., Kharouf, M., Gourabi, H., Hosseini, S.H., et al. (2019). Whole exome sequencing of men with multiple morphological abnormalities of the sperm flagella reveals novel homozygous QRICH2 mutations. Clin Genet 96, 394–401.
Kim, K., Ryu, S.M., Kim, S.T., Baek, G., Kim, D., Lim, K., Chung, E., Kim, S., and Kim, J.S. (2017). Highly efficient RNA-guided base editing in mouse embryos. Nat Biotechnol 35, 435–437.
Komor, A.C., Kim, Y.B., Packer, M.S., Zuris, J.A., and Liu, D.R. (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424.
Kuscu, C., Parlak, M., Tufan, T., Yang, J., Szlachta, K., Wei, X., Mammadov, R., and Adli, M. (2017). CRISPR-STOP: gene silencing through base-editing-induced nonsense mutations. Nat Methods 14, 710–712.
Lehti, M.S., Kotaja, N., and Sironen, A. (2013). KIF3A is essential for sperm tail formation and manchette function. Mol Cell Endocrinol 377, 44–55.
Lehti, M.S., and Sironen, A. (2016). Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 151, R43–R54.
Li, G., Li, X., Zhuang, S., Wang, L., Zhu, Y., Chen, Y., Sun, W., Wu, Z., Zhou, Z., Chen, J., et al. (2022). Gene editing and its applications in biomedicine. Sci China Life Sci 65, 660–700.
Li, J., Liu, Z., Huang, S., Wang, X., Li, G., Xu, Y., Yu, W., Chen, S., Zhang, Y., Ma, H., et al. (2019). Efficient base editing in G/C-rich regions to model androgen insensitivity syndrome. Cell Res 29, 174–176.
Litscher, E.S., and Wassarman, P.M. (2020). Zona pellucida proteins, fibrils, and matrix. Annu Rev Biochem 89, 695–715.
Liu, C., He, X., Liu, W., Yang, S., Wang, L., Li, W., Wu, H., Tang, S., Ni, X., Wang, J., et al. (2019a). Bi-allelic mutations in TTC29 cause male subfertility with asthenoteratospermia in humans and mice. Am J Hum Genet 105, 1168–1181.
Liu, C., Litscher, E.S., Mortillo, S., Sakai, Y., Kinloch, R.A., Stewart, C.L., and Wassarman, P.M. (1996). Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci USA 93, 5431–5436.
Liu, C., Miyata, H., Gao, Y., Sha, Y., Tang, S., Xu, Z., Whitfield, M., Patrat, C., Wu, H., Dulioust, E., et al. (2020). Bi-allelic DNAH8 variants lead to multiple morphological abnormalities of the sperm flagella and primary male infertility. Am J Hum Genet 107, 330–341.
Liu, C., Tu, C., Wang, L., Wu, H., Houston, B.J., Mastrorosa, F.K., Zhang, W., Shen, Y., Wang, J., Tian, S., et al. (2021a). Deleterious variants in X-linked CFAP47 induce asthenoteratozoospermia and primary male infertility. Am J Hum Genet 108, 309–323.
Liu, Y., DeBoer, K., de Kretser, D.M., O’Donnell, L., O’Connor, A.E., Merriner, D.J., Okuda, H., Whittle, B., Jans, D.A., Efthymiadis, A., et al. (2015). LRGUK-1 is required for basal body and manchette function during spermatogenesis and male fertility. PLoS Genet 11, e1005090.
Liu, Z., Chen, M., Chen, S., Deng, J., Song, Y., Lai, L., and Li, Z. (2018a). Highly efficient RNA-guided base editing in rabbit. Nat Commun 9, 2717.
Liu, Z., Chen, S., Jia, Y., Shan, H., Chen, M., Song, Y., Lai, L., and Li, Z. (2021b). Efficient and high-fidelity base editor with expanded PAM compatibility for cytidine dinucleotide. Sci China Life Sci 64, 1355–1367.
Liu, Z., Chen, S., Shan, H., Zhang, Q., Chen, M., Lai, L., and Li, Z. (2019b). Efficient and precise base editing in rabbits using human APOBEC3A-nCas9 fusions. Cell Discov 5, 31.
Liu, Z., Lu, Z., Yang, G., Huang, S., Li, G., Feng, S., Liu, Y., Li, J., Yu, W., Zhang, Y., et al. (2018b). Efficient generation of mouse models of human diseases via ABE- and BE-mediated base editing. Nat Commun 9, 2338.
Lorès, P., Dacheux, D., Kherraf, Z.E., Nsota Mbango, J.F., Coutton, C., Stouvenel, L., Ialy-Radio, C., Amiri-Yekta, A., Whitfield, M., Schmitt, A., et al. (2019). Mutations in TTC29, encoding an evolutionary conserved axonemal protein, result in asthenozoospermia and male infertility. Am J Hum Genet 105, 1148–1167.
Ma, Q., Cao, C., Zhuang, C., Luo, X., Li, X., Wan, H., Ye, J., Chen, F., Cui, L., Zhang, Y., et al. (2021). AXDND1, a novel testis-enriched gene, is required for spermiogenesis and male fertility. Cell Death Discov 7, 348.
Mehravar, M., Shirazi, A., Nazari, M., and Banan, M. (2019). Mosaicism in CRISPR/Cas9-mediated genome editing. Dev Biol 445, 156–162.
Netherton, J.K., Hetherington, L., Ogle, R.A., Velkov, T., and Baker, M.A. (2018). Proteomic analysis of good- and poor-quality human sperm demonstrates that several proteins are routinely aberrantly regulated. Biol Reprod 99, 395–408.
Oetting, W.S. (2000). The tyrosinase gene and oculocutaneous albinism type 1 (OCA1): a model for understanding the molecular biology of melanin formation. Pigment Cell Res 13, 320–325.
Rankin, T., Familari, M., Lee, E., Ginsberg, A., Dwyer, N., Blanchette-Mackie, J., Drago, J., Westphal, H., and Dean, J. (1996). Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 122, 2903–2910.
Sha, Y.W., Xu, X., Mei, L.B., Li, P., Su, Z.Y., He, X.Q., and Li, L. (2017). A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF). Gene 633, 48–53.
Shannon, P., Markiel, A., Ozier, O., Baliga, N.S., Wang, J.T., Ramage, D., Amin, N., Schwikowski, B., and Ideker, T. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504.
Shen, Y., Zhang, F., Li, F., Jiang, X., Yang, Y., Li, X., Li, W., Wang, X., Cheng, J., Liu, M., et al. (2019). Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat Commun 10, 433.
Song, R., Wang, Y., Zheng, Q., Yao, J., Cao, C., Wang, Y., and Zhao, J. (2022). One-step base editing in multiple genes by direct embryo injection for pig trait improvement. Sci China Life Sci 65, 739–752.
Tang, S., Wang, X., Li, W., Yang, X., Li, Z., Liu, W., Li, C., Zhu, Z., Wang, L., Wang, J., et al. (2017). Biallelic mutations in CFAP43 and CFAP44 cause male infertility with multiple morphological abnormalities of the sperm flagella. Am J Hum Genet 100, 854–864.
Thomas, L., Bouhouche, K., Whitfield, M., Thouvenin, G., Coste, A., Louis, B., Szymanski, C., Bequignon, E., Papon, J.F., Castelli, M., et al. (2020). TTC12 loss-of-function mutations cause primary ciliary dyskinesia and unveil distinct dynein assembly mechanisms in motile cilia versus flagella. Am J Hum Genet 106, 153–169.
Touré, A., Martinez, G., Kherraf, Z.E., Cazin, C., Beurois, J., Arnoult, C., Ray, P.F., and Coutton, C. (2021). The genetic architecture of morphological abnormalities of the sperm tail. Hum Genet 140, 21–42.
Tu, C., Cong, J., Zhang, Q., He, X., Zheng, R., Yang, X., Gao, Y., Wu, H., Lv, M., Gu, Y., et al. (2021). Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice. Am J Hum Genet 108, 1466–1477.
Vandenbrouck, Y., Pineau, C., and Lane, L. (2020). The functionally unannotated proteome of human male tissues: a shared resource to uncover new protein functions associated with reproductive biology. J Proteome Res 19, 4782–4794.
Wang, H., Yang, H., Shivalila, C.S., Dawlaty, M.M., Cheng, A.W., Zhang, F., and Jaenisch, R. (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918.
Wang, W., Tian, S., Nie, H., Tu, C., Liu, C., Li, Y., Li, D., Yang, X., Meng, L., Hu, T., et al. (2021). CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum Mol Genet 30, 2240–2254.
Wang, W., Tu, C., Nie, H., Meng, L., Li, Y., Yuan, S., Zhang, Q., Du, J., Wang, J., Gong, F., et al. (2019). Biallelic mutations in CFAP65 lead to severe asthenoteratospermia due to acrosome hypoplasia and flagellum malformations. J Med Genet 56, 750–757.
Wang, X., Li, J., Wang, Y., Yang, B., Wei, J., Wu, J., Wang, R., Huang, X., Chen, J., and Yang, L. (2018). Efficient base editing in methylated regions with a human APOBEC3A-Cas9 fusion. Nat Biotechnol 36, 946–949.
Wassarman, P.M., Jovine, L., and Litscher, E.S. (2001). A profile of fertilization in mammals. Nat Cell Biol 3, E59–E64.
Wassarman, P.M., and Litscher, E.S. (2022). Female fertility and the zona pellucida. eLife 11, e76106.
Wassarman, P.M., Liu, C., Chen, J., Qi, H., and Litscher, E.S. (1998). Ovarian development in mice bearing homozygous or heterozygous null mutations in zona pellucida glycoprotein gene mZP3. Histol Histopathol 13, 293–300.
Wei, W., and Gao, C. (2022). Gene editing: from technologies to applications in research and beyond. Sci China Life Sci 65, 657–659.
Whitfield, M., Thomas, L., Bequignon, E., Schmitt, A., Stouvenel, L., Montantin, G., Tissier, S., Duquesnoy, P., Copin, B., Chantot, S., et al. (2019). Mutations in DNAH17, encoding a sperm-specific axonemal outer dynein arm heavy chain, cause isolated male infertility due to asthenozoospermia. Am J Hum Genet 105, 198–212.
Wu, B., Yu, X., Liu, C., Wang, L., Huang, T., Lu, G., Chen, Z.J., Li, W., and Liu, H. (2021). Essential role of CFAP53 in sperm flagellum biogenesis. Front Cell Dev Biol 9, 676910.
Yan, W. (2009). Male infertility caused by spermiogenic defects: lessons from gene knockouts. Mol Cell Endocrinol 306, 24–32.
Yang, G., Zhu, T., Lu, Z., Li, G., Zhang, H., Feng, S., Liu, Y., Li, J., Zhang, Y., Chen, J., et al. (2018). Generation of isogenic single and multiplex gene knockout mice by base editing-induced STOP. Sci Bull 63, 1101–1107.
Yang, W., Qi, W., Li, Y., Wang, J., Luo, Y., Ding, D., Mo, S., Chen, B., Lu, Y., Li, H., et al. (2021). Programmed sequential cutting endows Cas9 versatile base substitution capability in plants. Sci China Life Sci 64, 1025–1028.
Yen, S.T., Zhang, M., Deng, J.M., Usman, S.J., Smith, C.N., Parker-Thornburg, J., Swinton, P.G., Martin, J.F., and Behringer, R.R. (2014). Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev Biol 393, 3–9.
Young, S., Miyata, H., Satouh, Y., Kato, H., Nozawa, K., Isotani, A., Aitken, R., Baker, M., and Ikawa, M. (2015). CRISPR/Cas9-mediated rapid generation of multiple mouse lines identified Ccdc63 as essential for spermiogenesis. Int J Mol Sci 16, 24732–24750.
Zhang, B., Khan, I., Liu, C., Ma, A., Khan, A., Zhang, Y., Zhang, H., Kakakhel, M.B.S., Zhou, J., Zhang, W., et al. (2021a). Novel loss-of-function variants in DNAH17 cause multiple morphological abnormalities of the sperm flagella in humans and mice. Clin Genet 99, 176–186.
Zhang, H., Pan, H., Zhou, C., Wei, Y., Ying, W., Li, S., Wang, G., Li, C., Ren, Y., Li, G., et al. (2018a). Simultaneous zygotic inactivation of multiple genes in mouse through CRISPR/Cas9-mediated base editing. Development 145, dev.168906.
Zhang, R., Chen, S., Meng, X., Chai, Z., Wang, D., Yuan, Y., Chen, K., Jiang, L., Li, J., and Gao, C. (2021b). Generating broad-spectrum tolerance to ALS-inhibiting herbicides in rice by base editing. Sci China Life Sci 64, 1624–1633.
Zhang, R., Wu, B., Liu, C., Zhang, Z., Wang, X., Wang, L., Xiao, S., Chen, Y., Wei, H., Jiang, H., et al. (2022). CCDC38 is required for sperm flagellum biogenesis and male fertility in mice. Development 149, dev200516.
Zhang, W., Aida, T., del Rosario, R.C.H., Wilde, J.J., Ding, C., Zhang, X., Baloch, Z., Huang, Y., Tang, Y., Li, D., et al. (2020). Multiplex precise base editing in cynomolgus monkeys. Nat Commun 11, 2325.
Zhang, Y., Liu, H., Li, W., Zhang, Z., Zhang, S., Teves, M.E., Stevens, C., Foster, J.A., Campbell, G.E., Windle, J.J., et al. (2018b). Intraflagellar transporter protein 140 (IFT140), a component of IFT-A complex, is essential for male fertility and spermiogenesis in mice. Cytoskeleton 75, 70–84.
Zheng, R., Sun, Y., Jiang, C., Chen, D., Yang, Y., and Shen, Y. (2021). A novel mutation in DNAH17 is present in a patient with multiple morphological abnormalities of the flagella. Reprod Biomed Online 43, 532–541.
Acknowledgement
This work was supported by the National Key Research and Development Program of China (2021YFC2701400) and the National Natural Science Foundation of China (32000393, 32322017, 32288101). We would like to thank the Center of Cryo-Electron Microscopy (CCEM) at Zhejiang University and Li Wang (CCEM) for their technical support on TEM. We would like to thank Professor Qinghua Shi (University of Science and Technology of China) and Chunyu Liu (Fudan University) for providing the anti-DNAH17 antibody. We would like to thank the Imaging Core Facility of State Key Laboratory of Genetic Engineering and the Facility of IMIB (Institute of Metabolism and Integrative Biology) at Fudan University for technical support. We would like to thank Zhicheng Wu (Tongji University) for support on piRNA analysis. We would like to thank Professor Hua Diao (Shanghai Institute for Biomedical and Pharmaceutical Technologies) for technical support on CASA analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no conflict of interest.
Supporting Information for
11427_2023_2408_MOESM1_ESM.pdf
Gene-knockout by iSTOP enables rapid reproductive disease modeling and phenotyping in germ cells of the founder generation
Rights and permissions
About this article
Cite this article
Wang, Y., Chen, J., Huang, X. et al. Gene-knockout by iSTOP enables rapid reproductive disease modeling and phenotyping in germ cells of the founder generation. Sci. China Life Sci. 67, 1035–1050 (2024). https://doi.org/10.1007/s11427-023-2408-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-023-2408-2